Clopidogrel: Personalized medicine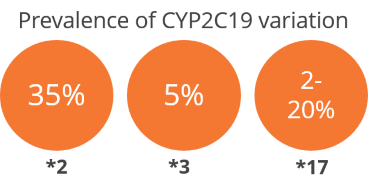
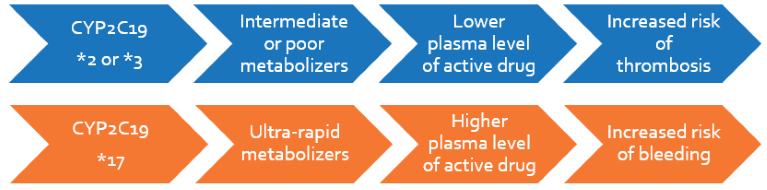
Clopidogrel is a platelet inhibitor used in patients with cardiovascular or cerebrovascular risk. It has a very narrow therapeutic index depending on the patient’s metabolism, which is influenced by CYP2C19. Based on the genotype, patients are classified from ultra-rapid (UM) to poor metabolizers (PM). PMs have increased risk of thrombotic events because of sub-therapeutic levels of the active metabolite, whereas UMs have increased risk of haemorrhage. The FDA has recommended CYP2C19 genotyping and pharmacogenetic dosing for patients who are administered clopidogrel.
Clinical Significance
- Patients are stratified based on the zygosity and number of different allelic variants
- Poor metabolizers (PMs) and intermediate metabolizers may require 2-4-fold higher dose to achieve therapeutic benefits and prevent clotting-related adverse events
- Ultra-rapid metabolizers (UMs) may require lower doses of clopidogrel or monitoring in order to prevent bleeding-related adverse events
Personalize Clopidogrel Test
Patients will be genotyped based on three highly prevalent polymorphisms that account for >99% of the population and classified as Ultra-rapid (UM), Extensive (EM), Intermediate (IM) or Poor metabolizers. The report will include recommendations on the need for switching to alternative platelet inhibitors, as well as suggestions for appropriate, tailored clopidogrel dosage.
 Guideline: Clinical Pharmacogenetics Implementation Consortium for Clopidogrel therapy, Scott SA et al. 2013, Clinical Pharmacology and Therapeutics
Guideline: Clinical Pharmacogenetics Implementation Consortium for Clopidogrel therapy, Scott SA et al. 2013, Clinical Pharmacology and Therapeutics
- Genotype-based anti-platelet choice and dosing are recommended for patients with Acute Coronary Syndrome (ACS) or undergoing Percutaneous Coronary Intervention (PCI)
- For patients with ACS or undergoing PCI, the risks for loss-of-function allele carriers were:
‐for major adverse cardiovascular events:
HR = 1.76, 95% CI = 1.24–2.50 for homozygotes
HR = 1.55, 95% CI = 1.11–2.17 for heterozygotes
‐for increased risks of stent thrombosis:
HR = 3.97, 95% CI = 1.75–9.02 for homozygotes
HR = 2.67, 95% CI = 1.69–4.22 for heterozygotes
- Prasugrel and ticagrelor were more beneficial than clopidogrel only in the loss-of-function carriers
Meta-analysis: Polymorphisms significantly associated with risk of adverse events for clopidogrel therapy, Mao L et al. 2013, Archives of Cardiovascular Diseases
- Meta-analysis of 21 studies including 23035 patients from prospective cohort or randomized, controlled trials was carried out
- Loss-of-function variants had increased risk of the following:
‐ischaemic stroke: OR 2.14, p <0.001
‐stent thrombosis: OR 2.08, p < 0.00001
‐myocardial infarction: OR 1.62, p < 0.00001
‐adverse clinical events: OR 1.50, p=0.0003
Meta-analysis: Significant risk for both loss- and gain-of-function carriers for cardiovascular outcomes in coronary artery disease, Zabalza M et al. 2012, Heart
- Loss of function *2 allele was associated with an increased risk of stent thrombosis: HR=2.24 (95% CI 1.52 to 3.30)
- Gain-of-function *17 allele was associated with the following:
‐higher risk of major bleeding: HR =1.26 (95% CI 1.05 to 1.50)
‐lower risk of cardiovascular events: HR =0.75 (95% CI 0.66 to 0.87)
Both loss- and gain-of-function carriers suffer increased mortality after myocardial infarction – TRIUMPH study, Cresci S et al. 2014, Circulation and Cardiovascular Genetics
- Prospective, multicentre study of 2732 patients hospitalized with acute myocardial infarction
- Among *2-allele carriers, significantly increased 1-year mortality (adjusted HR=1.7, p=0.046) and increased trend of recurrent MI in 1 year (adjusted HR=2.1, p=0.066) were observed in whites
- Among *17 gain-of-function allele carriers, significantly increased 1-year mortality (adjusted HR=8.97, p<0.0001) and bleeding episodes were observed
Quadrupling normal clopidogrel dose necessary in poor metabolizers to maintain effect, Horenstein RB et al. 2014, Journal of Clinical Pharmacology
- PMs needed 4-fold higher dose, and IMs needed a 2-fold higher dose than EMs at Day 8:
‐To attain similar maximal platelet aggregation 4 h post-dose
‐To achieve similar active metabolite concentration
