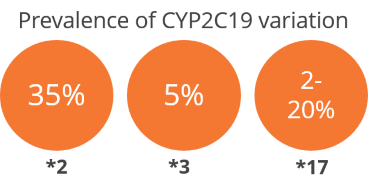 Precise dosing of Clopidogrel to prevent severe side-effects
Precise dosing of Clopidogrel to prevent severe side-effects
Individualized clopidogrel dosage helps avoid emergencies from morbid bleeding and clotting
60% of population faces high risk
Formation of a blood clot in the artery to the heart or the brain causes heart attack or stroke. Clopidogrel is the blood thinner that is given after such an event to prevent further occurrence of these events. The concentration of the active ingredient differs in each person. This is due to individual differences in metabolism caused by genetic variations. Up to 40% of the population are intermediate to poor metabolizers with increased risk of clotting or thrombotic events, such as stent thrombosis, on using clopidogrel. About 2-20% of the population are very rapid metabolizers who are at increased risk of internal bleeding or hemorrhage. The FDA has recommended testing for genotyping and pharmacogenetic dosing for administering Clopidogrel to patients.
Decrease risk of stent thrombosis or bleeding
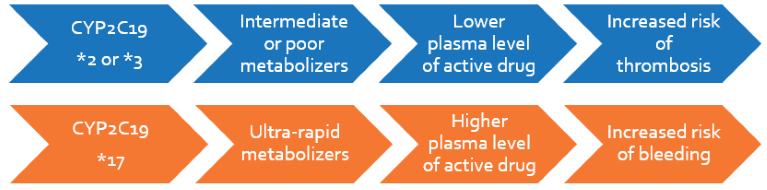
Each person has different rates of clopidogrel metabolism affecting their body’s response to the drug. Poor metabolizers and intermediate metabolizers may not respond to clopidogrel or may require 2-4 fold higher dose to prevent clotting-related side-effects. Alternatively, anti-platelets such as prasugrel or ticagrelor may be prescribed subject to contraindications.
Ultra-rapid metabolizers of the drug may require normal or lower doses of clopidogrel in order to prevent bleeding-related side-effects. Patients with normal metabolism may save costs using the less expensive clopidogrel drug at standard dosage.
Genetics of Clopidogrel metabolism, and FAQs
Clopidogrel is a ‘blood thinner’ that inhibits platelet aggregation, used in patients with cardiovascular or cerebrovascular risk. The safe concentration within which it brings about the desired effect is dependent on the patient’s metabolism. This is influenced by variations in the gene, CYP2C19 that is responsible for regulating clopidogrel metabolism in the body. Depending on their metabolism, patients that have slow or reduced activation of the drug face increased risk of thrombotic events, whereas those that activate the drug faster have increased risk of haemorrhage. The FDA has recommended CYP2C19 genotyping and pharmacogenetic dosing for patients who are administered clopidogrel.
The test categorizes individuals for clopidogrel metabolism by genotyping CYP2C19 *2, *3 and *17 using allele-specific real-time PCR. The method has almost 100% analytical accuracy and sensitivity. These three variants account for >99% of the population prevalence for CYP2C19.
References
Scott SA et al. 2013, Clinical Pharmacology and Therapeutics
Mao L et al. 2013, Archives of Cardiovascular Diseases
