Statins: Personalized medicine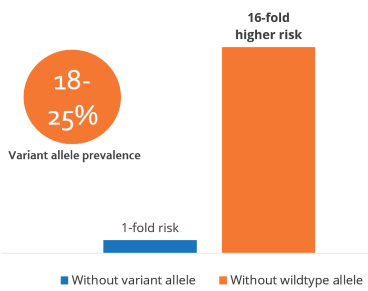
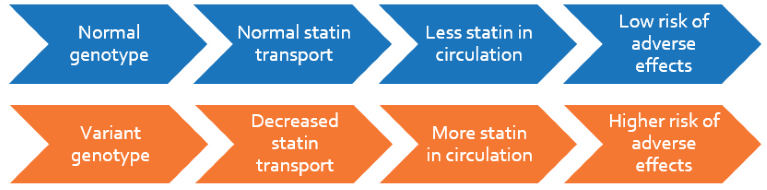
Despite the benefits of statin treatment in lowering cholesterol, statin-related adverse effects including myalgia and myopathy have been widely reported in patients. The incidence of muscle complaints in patients using statins is 9-20% in an outpatient setting. Statin pharmacokinetics is mediated by a transporter, SLCO1B1, that enables the hepatic absorption of statins from circulation. A common variation (521T>C) in this gene reduces the transporter activity leading to higher plasma statin levels and adverse effects.
Clinical Significance
- Patients carrying the variant allele are at higher risk of developing statin-induced muscle adversities with the risk increasing for every copy of the allele
- Over 1 out of 6 people are carriers of this variant
- For these patients, statin dosage may be lowered, alternative statins less likely to induce myopathy may be considered, or other dyslipidemic drugs may be substituted
Genotyping can thus help minimize adverse effects, improve treatment adherence and therefore efficacy
Personalize Statins Test
Patients beginning or on statin treatment will be genotyped for the SLCO1B1 521T>C variation and their risk of developing muscle-related side effects will be determined. For patients with the variant genotype, alternative treatment strategies may be indicated ranging from decreased statin dosage to switching to alternative statins with decreased risk for myopathy.
 Guideline: Clinical Pharmacogenetics Implementation Consortium on SLCO1B1 and simvastatin-induced myopathy, Ramsey LB et al. 2014, Clinical Pharmacology and Therapeutics
Guideline: Clinical Pharmacogenetics Implementation Consortium on SLCO1B1 and simvastatin-induced myopathy, Ramsey LB et al. 2014, Clinical Pharmacology and Therapeutics
- Individuals without variant can be given the desired simvastatin starting dose, while those with one or two copies of the variant should be given a lower dose or an alternative statin
- Frequency of adverse drug reactions increases with the co-administration of medications that alter simvastatin pharmacokinetics
Increased risk of myopathy in statin-treated, variant allele carriers, Carr DF et al. 2013, Clinical Pharmacology and Therapeutics
- A meta-analysis of 7 studies associating the risk of the variant allele with myopathy in 60,000 individuals taking various statins
- Risk of myopathy in statin users was significantly increased in the presence of variant allele.
- Odds ratio for myopathy was found to be:
– Overall: 2.18 (1.39-3.43)
– Simvastatin users: 3.25 (1.72-6.12)
– Atorvastatin users: 1.54 (0.80–2.97)
Significant association of the variant allele with myopathy in simvastatin users – SEARCH study, Link E et al. 2008, The New England Journal of Medicine
- A genome-wide association study screened 300,000 markers in 85 individuals with definite or incipient myopathy and 90 controls, all of whom were on simvastatin treatment
- More than 60% of the myopathy cases could be attributed to the presence of the variant allele
- Odds ratio for developing myopathy depended on the number of variant alleles:
‐One copy: 4.5 (95% CI, 2.6 – 7.7)
‐Two copies: 16.9 (95% CI, 4.7 – 61.1)
Effect of the variant allele on drug dosage and treatment adherence, De Keyser CE et al. 2014, Pharmacogenetics and Genomics
- The variant allele was associated with a decrease in statin dosage or switching over to other cholesterol-lowering drugs (indicators of adverse events) in 1939 incident simvastatin and atorvastatin users
- The risk of treatment modification was higher in individuals homozygous for the variant alleles than wildtypes in simvastatin users (HR 1.74, 95% CI 1.05 – 2.88) and in atorvastatin users starting at a dose more than 20mg (HR 3.26, 95% CI 1.47 – 7.25)
Effect of the variant allele on statin-induced side effects – STRENGTH study, Voora D et al. 2009, Journal of the American College of Cardiology
- Composite adverse effects (CAE), which included creatine kinase levels >3*baseline, myalgia or discontinuation for any side effects, were measured in 452 hypercholesterolemia patients randomized to receive atorvastatin 10 mg, simvastatin 20 mg, or pravastatin 10 mg followed by 80 mg, 80 mg, and 40 mg, respectively
- 37% variant allele carriers developed CAE and 25% did not (p = 0.03)
- A gene-dose effect was seen: percent with CAE in those with 0, 1 or 2 variant alleles were 19%, 27% and 50%
