Warfarin: Personalized medicine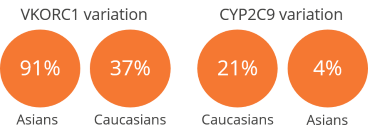
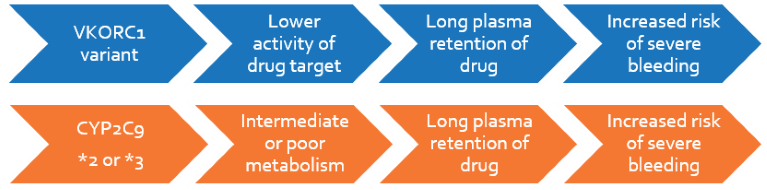
Warfarin (coumarin), acenocoumarol and phenprocoumon are vitamin K antagonist (VKA) anticoagulants used in patients with deep vein thrombosis, thromboembolism, cardiovascular or cerebrovascular risk, or after surgery. It has a very narrow therapeutic index and causes severe adverse events outside this range, being one of the most common reasons for emergency hospitalizations. Based on a patient’s VKORC1 and CYP2C9 genotypes, the dosage of warfarin may be adjusted to arrive sooner at the intended blood concentration, achieve therapeutic benefits, and minimize adverse effects. The majority of genetic dependence lies on VKORC1, which encodes a protein that is inhibited by warfarin, and CYP2C9, which is involved in warfarin metabolism. In 2010, FDA recommended pharmacogenetic warfarin dosing for patients based on VKORC1 and CYP2C9 genotypes.
Clinical Significance
- The loss-of-function allele prevalence may be as high as 95% in the Asian population:
- Prevalence for VKORC1: 91% in Asians, 37% in Caucasians
- Prevalence for CYP2C9: 21% in Caucasians, 4% in Asians
- Warfarin being one of the most common causes for emergency hospitalizations, it is critical to quickly and safely achieve the therapeutic concentrations for individuals on the drug
- Genotype-based warfarin dosing results in significantly rapid achievement of target INR compared to clinically-derived dosing algorithms
Personalize Warfarin Test
Patients will be genotyped for the two polymorphisms in CYP2C9 and VKORC1 1173C>T that are responsible for decreased therapeutic availability of the drug. The latter gene has a substantially higher effect on the target international normalized ratio (INR). The report will include recommendations on genotype-based warfarin dosing for tailored therapy.
 Guideline: Clinical Pharmacogenetics Implementation Consortium for Warfarin dosing, Johnson JA et al. 2011, Clinical Pharmacology and Therapeutics
Guideline: Clinical Pharmacogenetics Implementation Consortium for Warfarin dosing, Johnson JA et al. 2011, Clinical Pharmacology and Therapeutics
- Recommendations for dosing based on genetics are ‘Strong’ or ‘level-A’
- Genetics-based algorithms for dosing are even better than the FDA-recommended genotype-based table.
- Clinical INR-based dosing is iterative and takes weeks to months to arrive at the optimal therapeutic range resulting in significantly increased risk for over- or under-coagulation and thereby, severe thromboembolism or bleeding
Meta-analysis: Greater than 50% reduction in bleeding events upon pharmacogenetic VKA dosing, Franchini M et al. 2014, Journal of Thrombosis and Haemostasis
- Meta-analysis of nine randomized controlled trials with 2812 patients were pooled for comparing genotype-guided vs. clinically-guided dosing of VKAs
- The pharmacogenetic-guided group had >50% lower risk for developing major bleeding events compared to the clinically-guided group (RR = 0.47; 95% CI, 0.23-0.96; p = 0.04)
- When pharmacogenetic dosing algorithm is used, fewer than 1 in 2 patients suffered major bleeding effects of VKAs compared to dosage using clinical factors alone
Reduced hospitalization rates on genotype -based warfarin dosing – MM-WES study, Epstein RS et al. 2010, Journal of the American College of Cardiology
- Study of 6-month incidence of hospitalization in 896 patients receiving genotype-based warfarin dosage and 2688 patients receiving clinically-based dosage
- Compared to a simultaneous control cohort, genotyped cohort had:
‐43% lower risk of hospitalization for thromboembolism or bleeding (p = 0.003)
‐33% lower risk of overall hospitalization (p < 0.001)
- Compared with historical controls over 6-months, the genotyped cohort had:
‐31% fewer overall hospitalization (p < 0.001)
‐28% fewer hospitalization for thromboembolism or bleeding (p < 0.029)
Genetic warfarin dosing significantly superior to clinical- and FDA genetic table-guided dosing, Finkelman BS et al. 2011, Journal of the American College of Cardiology
- Retrospective cohort study of 1378 patients’ warfarin dose based on 2 clinically-guided tables, 2 genetic-guided tables and 1 pharmacogenetic algorithm
- Pharmacogenetic algorithm dosing was significantly more accurate (p < 0.001) than all other methods at achieving target INR
- Compared to pharmacogenetic-algorithm dosing
‐Clinical dosing had an odds ratio for adverse events of 2.2
‐genetic-table dosing had an odds ratio for adverse events of 1.8-1.9
Meta-analysis: VKORC1 polymorphism significantly associated with warfarin dose requirement, Jin B et al. 2014, Current Medical Research Opinion
- Meta-analysis of 32 prospective clinical trials involving 5005 patients
- On average, 2.62 mg/d dosage variation was required between wildtype and variant allele carriers (95% CI -3.10 to -2.14; P<0.00001)
- Significantly lower warfarin dose requirement was also observed in the heterozygotes (95% CI -1.67 to -0.96; P<0.00001)
